| 产品名称 |
Recombinant Thermobifida Fusca Cutinase (C-6His) |
| 英文名称 |
Cutinase |
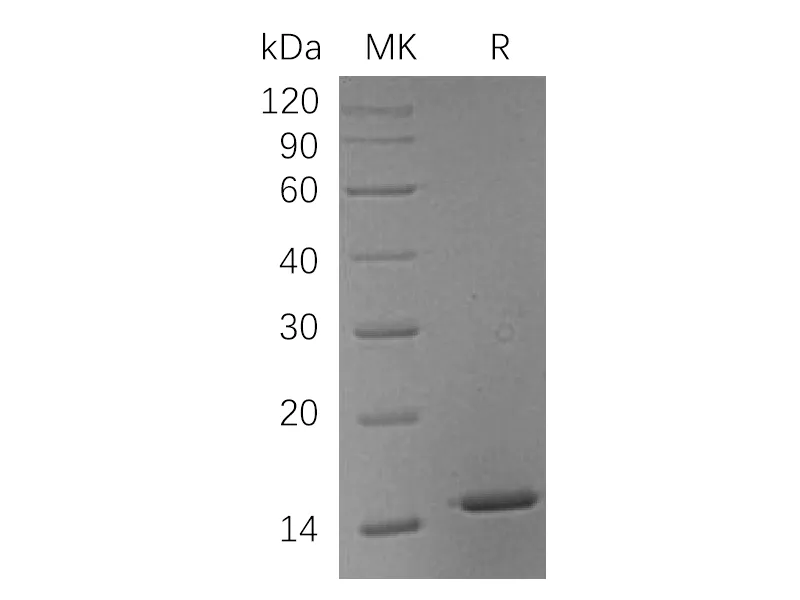
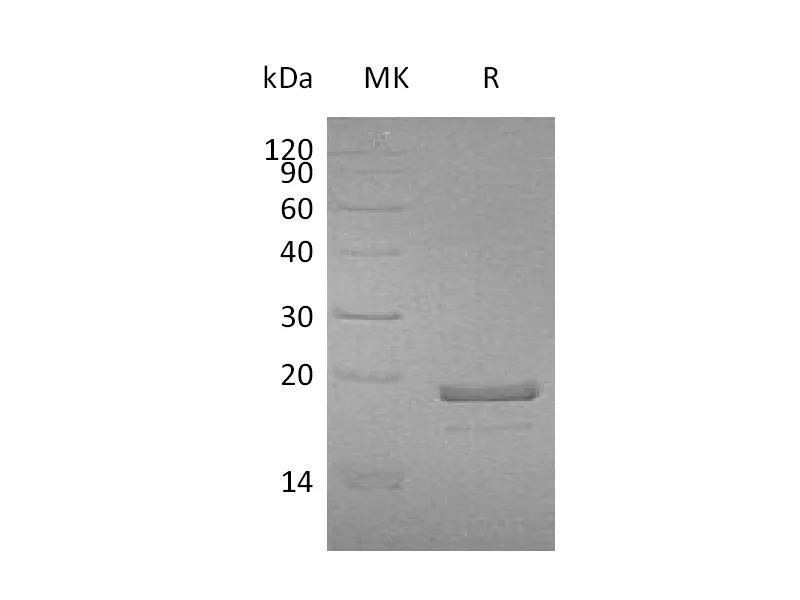
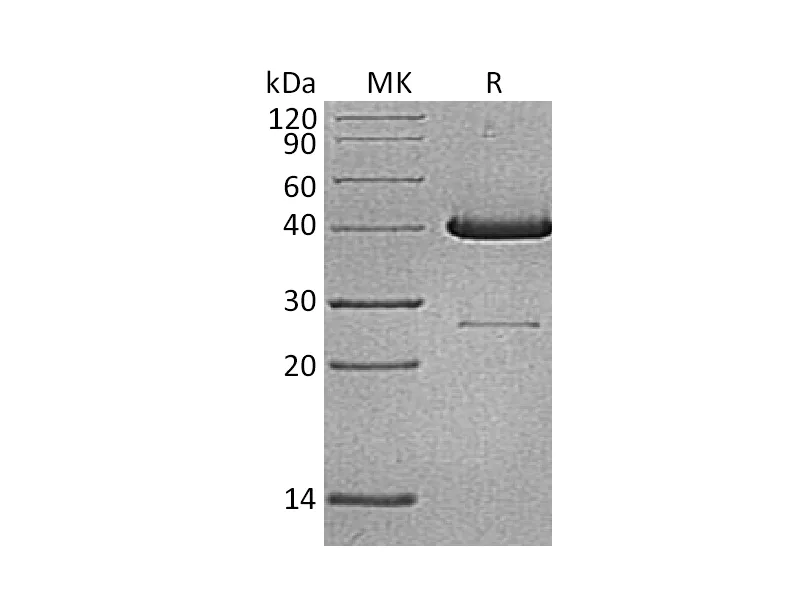
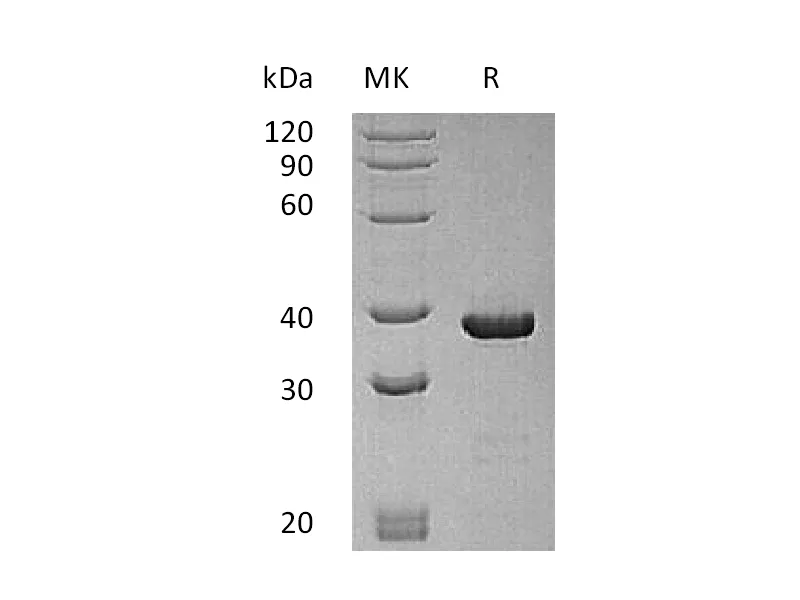
| 纯度 |
Greater than 95% as determined by reducing SDS-PAGE |
| 内毒素 |
<1 EU/µg as determined by LAL test. |
| 蛋白构建 |
Recombinant Thermobifida Fusca Cutinase is produced by our E.coli expression system and the target gene encoding Ala1-Phe261 is expressed with a 6His tag at the C-terminus. |
| Accession |
E5BBQ3 |
| 表达宿主 |
E.coli |
| 种属 |
Thermobifida fusca |
| 预测分子量 |
29.5 KDa |
| 制剂 |
Supplied as a 0.2 μm filtered solution of 20mM HAc-NaAc, 50% Glycerol, 5% Mannitol, 0.02% Tween 80, pH4.5. |
| 运输方式 |
The product is shipped on dry ice/polar packs.Upon receipt, store it immediately at the temperature listed below. |
| 稳定性&储存 |
Store at ≤-70°C, stable for 6 months after receipt.Store at ≤-70°C, stable for 3 months under sterile conditions after opening. Please minimize freeze-thaw cycles. |
| 复溶 |
| 分子别名 |
| Cutinase |
| 背景介绍 |
| Cutinase belongs to the family of hydrolases, specifically those acting on carboxylic ester bonds. The systematic name of this enzyme class is cutin hydrolase. Cutinase is a serine esterase containing the classical Ser, His, Asp triad of serine hydrolases. The protein belongs to the alpha-beta class, with a central beta-sheet of 5 parallel strands covered by 5 helices on either side of the sheet. Cutin monomers released from the cuticle by small amounts of cutinase on fungal spore surfaces can greatly increase the amount of cutinase secreted by the spore. The active site cleft is partly covered by 2 thin bridges formed by amino acid side chains, by contrast with the hydrophobic lid possessed by other lipases. The protein also contains 2 disulfide bridges, which are essential for activity, their cleavage resulting in complete loss of enzymatic activity. |
注意事项
本司产品仅用于科研,不用于临床诊断和治疗