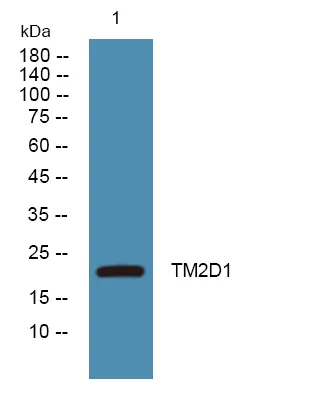
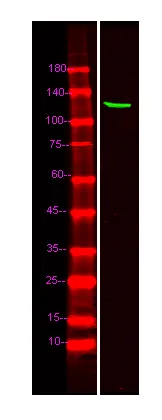
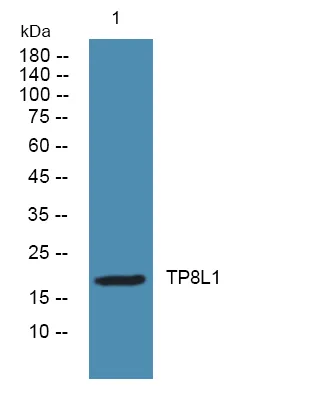
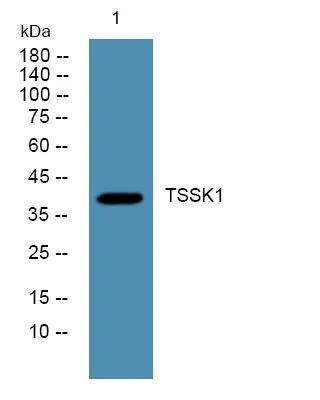
产品概述
产品性能
免疫原
产品应用
研究背景
Chromatin assembly factor I (CAF1) is a nuclear complex consisting of p50, p60 (CHAF1B; MIM 601245), and p150 (CHAF1A) subunits that assembles histone octamers onto replicating DNA in vitro (Kaufman et al., 1995 [PubMed 7600578]).[supplied by OMIM, Mar 2008],alternative products:Experimental confirmation may be lacking for some isoforms,developmental stage:Active complex is found in G1, S and G2 phases.,domain:Contains one Pro-Xaa-Val-Xaa-Leu (PxVxL) motif, which is required for interaction with chromoshadow domains. This motif requires additional residues -7, -6, +4 and +5 of the central Val which contact the chromoshadow domain.,function:Core component of the CAF-1 complex, a complex thought to mediate chromatin assembly in DNA replication and DNA repair. Assembles histone octamers onto replicating DNA in vitro. CAF-1 performs the first step of the nucleosome assembly process, bringing newly synthesized histones H3 and H4 to replicating DNA; histones H2A/H2B can bind to this chromatin precursor subsequent to DNA replication to complete the histone octamer. CHAF1A binds to histones H3 and H4. It may play a role in heterochromatin maintenance in proliferating cells by bringing newly synthesized cbx proteins to heterochromatic DNA replication foci (By similarity). The CCR4-NOT complex functions as general transcription regulation complex. Also involved in vitamin D-coupled transcription regulation via its association with the WINAC complex, a chromatin-remodeling complex recruited by vitamin D receptor (VDR), which is required for the ligand-bound VDR-mediated transrepression of the CYP27B1 gene.,PTM:Phosphorylated upon DNA damage, probably by ATM or ATR.,sequence caution:Contaminating sequence. Potential poly-A sequence starting in position 426.,sequence caution:Translation N-terminally extended.,similarity:Belongs to the CHAF1A family.,subcellular location:DNA replication foci.,subunit:Homodimer. Part of the CAF-1 complex that contains RBBP4, CHAF1B and CHAF1A. CHAF1A binds directly to CHAF1B. Only minor amounts of RBBP4 are complexed with CHAF1A and CHAF1B in G1 phase. Part of the CCR4-NOT core complex that contains CHAF1A, CHAF1B, CNOT1, CNOT2, CNOT3, CNOT4, CNOT6 and CNOT8. CHAF1A binds directly to PCNA and to CBX1. Binds MBD1. Interacts directly with CBX5 via the PxVxL motif. During DNA replication, it forms a S phase-specific complex that facilitates DNA methylation and histone H3 'Lys-9' methylation during replication-coupled chromatin assembly and is at least composed of the CHAF1A, MBD1 and SETDB1. Component of the WINAC complex, at least composed of SMARCA2, SMARCA4, SMARCB1, SMARCC1, SMARCC2, SMARCD1, SMARCE1, ACTL6A, BAZ1B/WSTF, ARID1A, SUPT16H, CHAF1A and TOP2B.,
研究领域
Epigenetics and Nuclear Signaling