| 产品名称 |
Recombinant Human HBQ1 (N-6His) |
| 英文名称 |
HBQ1/Hemoglobin subunit theta-1 |
| 纯度 |
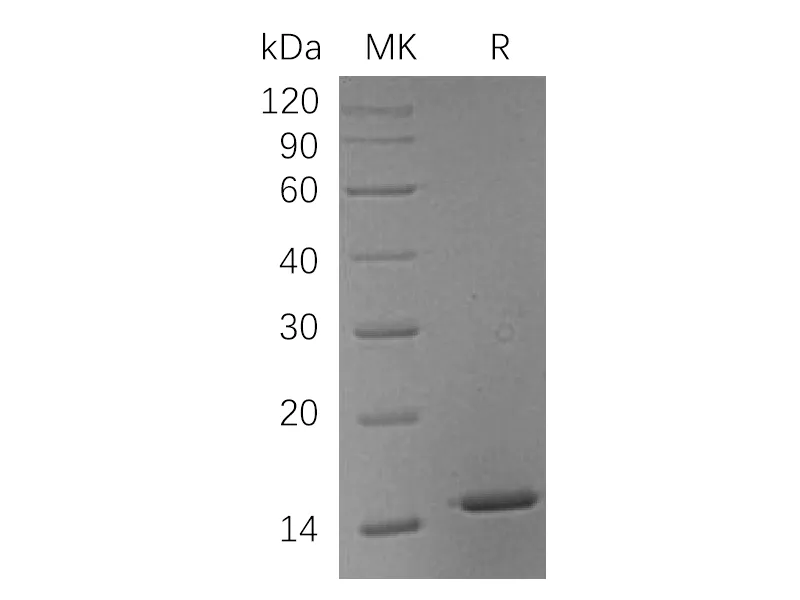
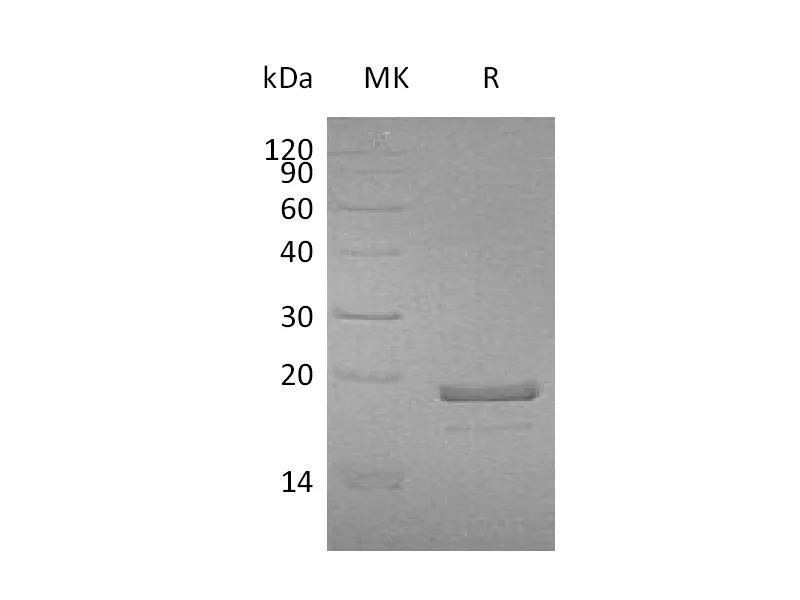
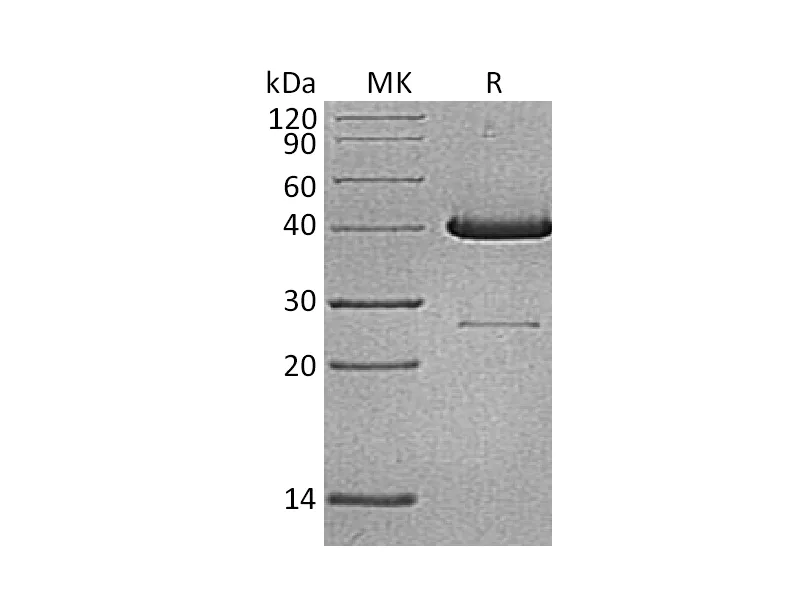
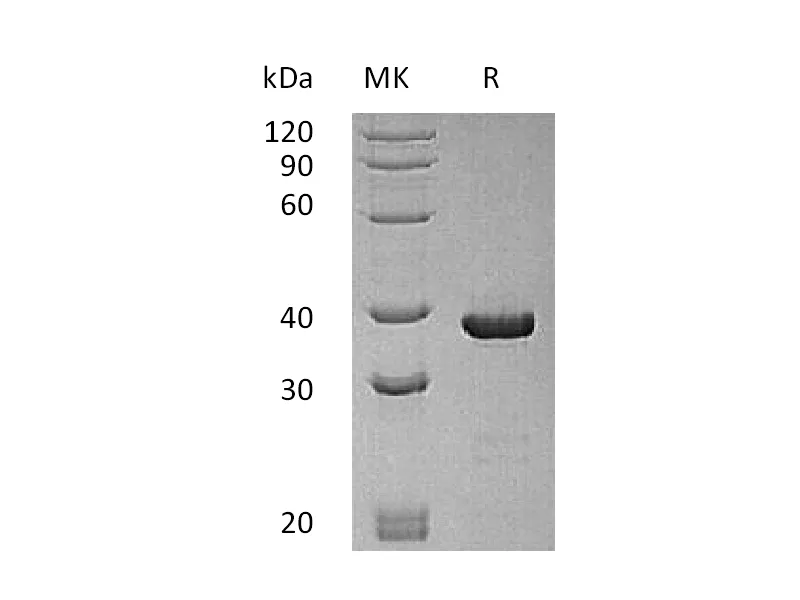
Greater than 95% as determined by reducing SDS-PAGE |
| 内毒素 |
<1 EU/µg as determined by LAL test. |
| 蛋白构建 |
Recombinant Human Hemoglobin Subunit Theta-1 is produced by our E.coli expression system and the target gene encoding Met1-Arg142 is expressed with a 6His tag at the N-terminus. |
| Accession |
P09105 |
| 表达宿主 |
E.coli |
| 种属 |
Human |
| 预测分子量 |
17.7 KDa |
| 制剂 |
Lyophilized from a 0.2 μm filtered solution of 20mM Histidine, 10% Sucrose, 3% Mannitol, 0.5mM EDTA, 0.05% Tween80, pH 5.5. |
| 运输方式 |
The product is shipped at ambient temperature.Upon receipt, store it immediately at the temperature listed below. |
| 稳定性&储存 |
Store at ≤-70°C, stable for 6 months after receipt.Store at ≤-70°C, stable for 3 months under sterile conditions after opening. Please minimize freeze-thaw cycles. |
| 复溶 |
Always centrifuge tubes before opening.Do not mix by vortex or pipetting.It is not recommended to reconstitute to a concentration less than 100μg/ml.Dissolve the lyophilized protein in distilled water.Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| 分子别名 |
| Hemoglobin subunit theta-1; Hemoglobin theta-1 chain; Theta-1-globin; HBQ1 |
| 背景介绍 |
| Hemoglobin subunit theta-1 is a protein that in humans is encoded by the HBQ1 gene. Theta-globin mRNA is originally found in human fetal erythroid tissue but not in adult erythroid or other nonerythroid tissue. Theta-1 is a member of the human alpha-globin gene cluster that includes five functional genes and two pseudogenes. Research supports a transcriptionally active role for the gene and a functional role for the peptide in specific cells, possibly those of early erythroid tissue. Hemoglobin has a quaternary structure characteristically composed of many multi-subunit globular proteins. Most of the amino acids in hemoglobin form alpha helices, connected by short non-helical segments. Hydrogen bonds stabilize the helical sections inside this protein, causing attractions within the molecule, folding each polypeptide chain into a specific shape. Hemoglobins quaternary structure comes from its four subunits in roughly a tetrahedral arrangement. |
注意事项
本司产品仅用于科研,不用于临床诊断和治疗