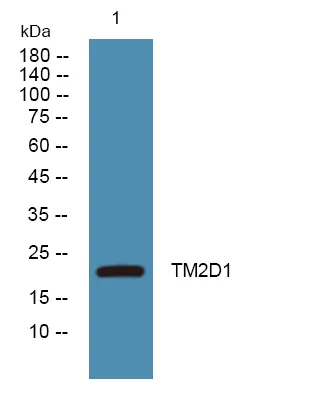
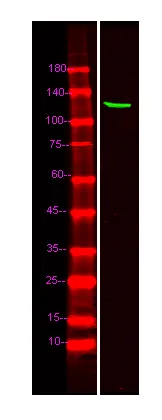
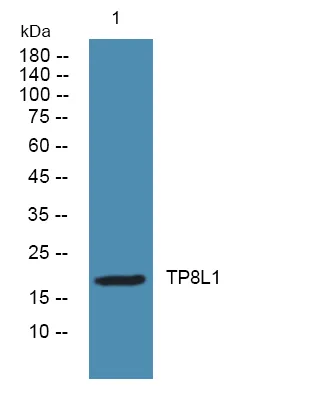
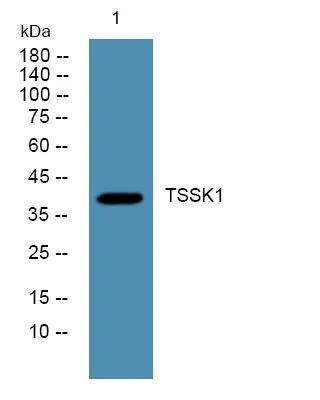
产品概述
产品性能
免疫原
产品应用
研究背景
domain:These WW domains interact with Arg/Gly-rich-flanked Pro-rich domains found in several WW domain-binding proteins (WBPs). The N-terminal WW domain has the greater ligand-binding ability.,PTM:Phosphorylated upon DNA damage, probably by ATM or ATR.,similarity:Contains 2 WW domains.,subunit:Binds FMN1. Interacts with the Arg/Gly-rich-flanked Pro-rich of KHDRBS1/SAM68. Arginine methylation in these regions has no effect on this binding.,domain:These WW domains interact with Arg/Gly-rich-flanked Pro-rich domains found in several WW domain-binding proteins (WBPs). The N-terminal WW domain has the greater ligand-binding ability.,PTM:Phosphorylated upon DNA damage, probably by ATM or ATR.,similarity:Contains 2 WW domains.,subunit:Binds FMN1. Interacts with the Arg/Gly-rich-flanked Pro-rich of KHDRBS1/SAM68. Arginine methylation in these regions has no effect on this binding.,
研究领域