产品概述
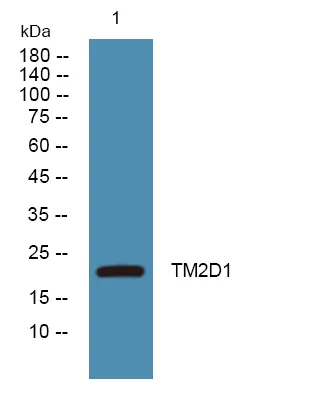
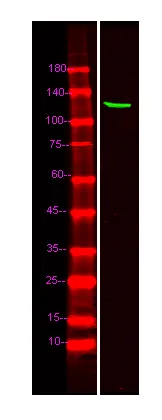
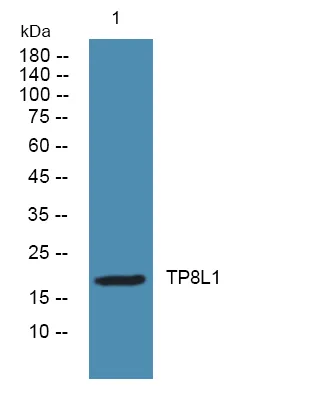
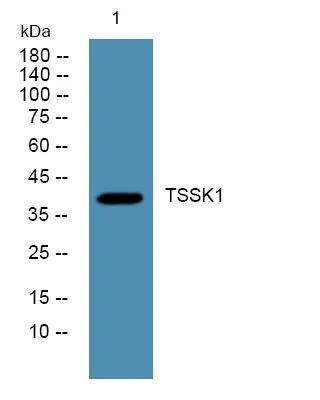
产品性能
免疫原
产品应用
研究背景
Several proteins have been found to be prenylated and methylated at their carboxyl-terminal ends. Prenylation was initially believed to be important only for membrane attachment. However, another role for prenylation appears to be its importance in protein-protein interactions. The only nuclear proteins known to be prenylated in mammalian cells are prelamin A- and B-type lamins. Prelamin A is farnesylated and carboxymethylated on the cysteine residue of a carboxyl-terminal CaaX motif. This post-translationally modified cysteine residue is removed from prelamin A when it is endoproteolytically processed into mature lamin A. The protein encoded by this gene binds to the prenylated prelamin A carboxyl-terminal tail domain. It may be a component of a prelamin A endoprotease complex. The encoded protein is located in the nucleus, where it partially colocalizes with the nuclear lamina. ItPTM:Phosphorylated upon DNA damage, probably by ATM or ATR.,similarity:Belongs to the NARF family.,subunit:Interacts with LMNA and binds to the farnesylated C-terminal domain.,tissue specificity:Ubiquitous. Predominantly expressed in skeletal muscle, heart and brain.,
研究领域
Transcription; Domain Families; Zinc Finger; Cell Biology; Proteolysis / Ubiquitin; Proteasome / Ubiquitin; Ubiquitin E3 Enzymes; RING Finger E3 Ligase; Epigenetics and Nuclear Signaling; Cell cycle; Ubiquitin ligases